Abstract
Background: Chimeric antigen receptor (CAR) T-cell therapy is a rapidly emerging form of treatment for hematologic malignancies including lymphoma, multiple myeloma, and leukemia. Hospitalizations and readmissions after CAR T-cell therapy have not been systematically studied. A better understanding of hospital utilization patterns could inform design of clinical trials, advanced planning of hospitalizations, timing of discharge, and frequency and type of outpatient follow-up.
Methods: We conducted a retrospective analysis of all patients admitted to the Massachusetts General Hospital for CAR T-cell therapy between 3/2016 and 3/2018. The primary outcome was hospital readmission within 30 days following discharge from CAR-T treatment visit. Secondary outcomes were ICU admission and inpatient mortality. Exploratory analyses were also conducted to determine whether patient age, length of initial hospitalization, laboratory measurements including ferritin and C-reactive protein (CRP), and tocilizumab exposure were associated with 30-day readmission. Summary statistics were reported by disease types, CAR-T products, and the primary and secondary outcomes using appropriate statistical functions. Comparison of laboratory values (ferritin and CRP) between the index admission (CAR T infusion) and first-readmission visits were analyzed using random intercept linear mixed effects models. Pairwise comparisons regarding mean difference of laboratory measurements during index admission and 30-day readmission/non-readmission were conducted and 95% confidence intervals reported. Bonferroni method was used for p-value adjustment (number of comparison groups = 4). This study was approved by the Partners Healthcare Institutional Review Board.
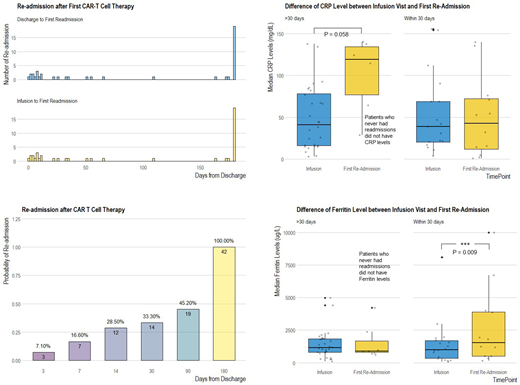
Results: Forty-two patients were treated with CAR T-cells between 3/2016 and 3/2018. Thirty-six patients had non-Hodgkins lymphoma (NHL), 1 acute lymphoblastic leukemia, 1 chronic lymphocytic leukemia, and 4 multiple myeloma. Eight (19%) received the standard of care product, axicabtagene ciloleucel and all others were treated on a clinical trial with an investigational product. Median age at first treatment was 62.2 years old. Twenty-nine patients were male and 13 female. Fourteen patients (33.3%) experienced readmission within 30 days following discharge from CAR T infusion hospitalization. Twelve patients (29%) were readmitted within the first 14 days and 20 patients (47.6%) were readmitted to the hospital within 90 days of discharge (Figure 1). Patient age was not associated with 30-day readmission (P=0.642). Thirty-four (81%) patients received one CAR-T treatment and 8 (19%) received 2 treatments. Among those who received a second CAR-T treatment, 4 (50%) experienced 30-day readmission. Overall, 3 (7%) patients required transfer to the ICU (1 patient after both CAR T infusions) and two patients with NHL died during their first CAR T treatment admission. One patient died of progressive disease 44 days post-CAR T and the other died of disseminated candidemia 18 day post-CAR T. Our mixed effects model showed that median ferritin level was significantly elevated during the 30-day readmission visit as compared to initial CAR T infusion visit (1526.5 vs 1005.5 ug/L, difference in mean = 1088.5, 95% CI: 437.4 to 1739.6, adjusted P = 0.009). CAR T infusion visit ferritin levels did not differ among patients who were readmitted within 30 days and those who were not (1143.0 vs 1005.5 ug/L, P = 1.0). For readmissions which occurred after 30 days, we did not find a statistically significant increase in ferritin levels (P = 0.809). We did not find an association between initial length of hospitalization, CRP levels, and tocilizumab usage and early readmissions (all P-values > 0.05).
Conclusions: CAR T-cell therapy is a promising evolving therapy for the treatment of relapsed, refractory hematologic malignancies. Further evaluation of pooled data may allow for early identification of patterns of deterioration which may limit premature hospital discharge, early readmission, and therapy-associated mortality.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.